Abstract
Background: IDH2 mutations are reported in ~12% of patients with AML. Enasidenib (AG-221) is a novel, small-molecule, oral inhibitor of mIDH2 proteins. The clinical efficacy of enasidenib is derived in part by differentiation of immature leukemic cells. Unlike cytotoxic therapies, differentiating agents can induce first responses months after treatment initiation. We report response and survival outcomes for patients with R/R AML in the phase 1/2 AG221-C-001 study (NCT01915498) who received enasidenib 100 mg daily and maintained stable disease (SD) during early treatment.
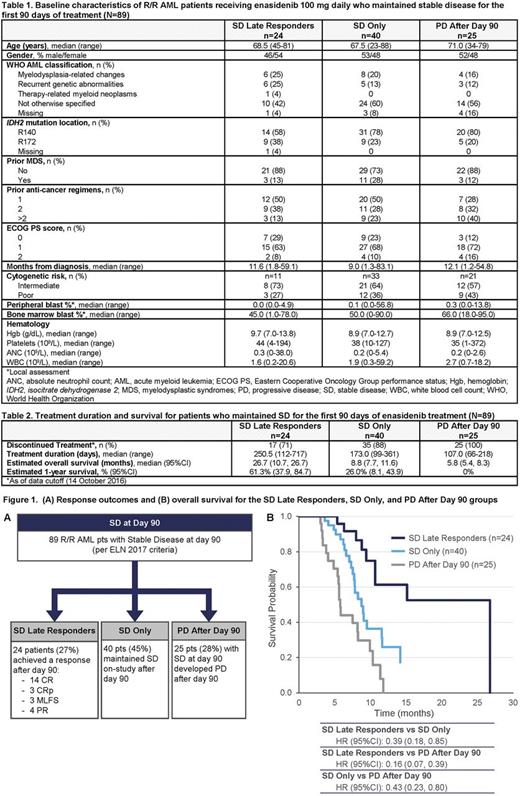
Methods: Analyses included patients with m IDH2 R/R AML who received enasidenib 100 mg daily. The "SD" cohort comprised patients who maintained SD per European LeukemiaNet (ELN) 2017 criteria; ie, had no formal IWG response and no evidence of progressive disease (PD) for at least 90 days. For these analyses, patients who maintained SD for the first 90 days on-study are divided into 3 subgroups: those who at any later time attained a response ("SD Late Responders"), those who continued to maintain SD after day 90 ("SD Only"), and those who progressed after day 90 ("PD After Day 90"). Kaplan-Meier estimated overall survival (OS) and 1-year survival rates are compared among the SD Late Responders, SD Only, and PD After Day 90 groups.
Results: At data cutoff (14 October 2016), among all 214 patients with m IDH2 R/R AML who received enasidenib 100 mg daily, 89 patients (41.6%) maintained SD for the first 90 days of treatment and comprised the SD cohort (Figure 1A). Of all SD patients, 24 (27%) became Late Responders, 40 patients (45%) continued to maintain SD Only, and 25 patients (28%) comprised the PD After Day 90 group. In univariate analyses, no baseline variable (Table 1)was significantly predictive of future response/non-response among SD patients.
SD Late Responders : 24 patients responded after day 90, 14 of whom achieved complete remission (Figure 1A). Median treatment duration for SD Late Responders was 250.5 days (range 112-717) (Table 2). Median time to first response was 129.5 days (range 90-336) and to best response was 140 days (90-341). Median OS was 26.7 months (95%CI 10.7, 26.7) and estimated 1-year survival rate was 61.3% (95%CI 37.9, 84.7).
SD Only : Median treatment duration for the 40 patients who continued to maintain SD after day 90 was 173 days (range 99-361). Median OS was 8.8 months (95%CI 7.7, 11.6) and estimated 1-year survival was 26.0% (95%CI 8.1, 43.9).
PD After Day 90 : Median treatment duration in this group was 107 days (range 66-218). Median OS for SD patients who progressed after day 90 was 5.8 months (95%CI 5.4, 8.3) and estimated 1-year survival was 0%.
Survival Comparisions : Median OS for all 89 pts who maintained SD for the first 90 treatment days was 9.0 months (95%CI 8.2, 11.4). Risk of death was significantly reduced in SD Late Responders by 61% vs the SD Only cohort (hazard ratio [HR] 0.39; 95%CI 0.18, 0.85) and by 84% vs the PD After Day 90 cohort (HR 0.16; 95%CI 0.07, 0.39) (Figure 1B). In the SD Only cohort, risk of death was reduced by 57% vs the PD After Day 90 cohort (HR 0.43; 95%CI 0.23, 0.80).
Conclusions: SD may represent sustained but controlled proliferation of leukemic cells that, in some cases, later differentiate and lead to clinical responses. In the first 90 days of treatment with enasidenib 100 mg daily, 42% of patients with m IDH2 R/R AML maintained SD. Of them, 1 in 4 responded after day 90, with median times to first and best responses of ~4 and ~5 months from treatment start. SD Late Responders had a significant OS benefit compared with patients who did not respond after day 90, and the estimated median OS for Late Responders-albeit a small group-was more than 2 years. R/R AML patients who maintained SD at all response evaluations (SD Only) received a median of ~6 months of enasidenib treatment and had a median OS of ~9 months, with a significant 57% reduction in risk of death vs patients with PD After Day 90. These data suggest that patients who sustain SD during early enasidenib treatment should continue treatment for at least 6 cycles or until disease progression. While no baseline factor was significantly predictive of a later response after sustained SD at day 90, results of ongoing molecular and translational analyses may be more informative. SD during early treatment with enasidenib does not predict treatment failure, and patients who maintain SD may benefit from continuing enasidenib treatment.
Stein: Constellation Pharma: Research Funding; Seattle Genetics: Research Funding; Novartis: Consultancy, Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Research Funding; GSK: Other: Advisory Board, Research Funding; Celgene Corporation: Consultancy, Other: Travel expenses, Research Funding; Pfizer: Consultancy, Other: Travel expenses. Stone: DSMN: Consultancy; Sumitomo Dainippon: Consultancy; Roche: Consultancy; Pfizer: Consultancy; Ono: Consultancy; Novartis: Consultancy; Juno Therapeutics: Consultancy; Jazz,: Consultancy; Janssen: Consultancy; Cornerstone: Consultancy; Astellas: Consultancy; Amgen: Consultancy; Agios: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Pollyea: Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis: Membership on an entity's Board of Directors or advisory committees; Agios, Pfizer: Research Funding. Roboz: AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace: Consultancy; Cellectis: Research Funding. Altman: Syros: Consultancy; NCCN: Other: Educational speaker; BMS: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Ceplene: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy; ASH: Other: Educational speaker. DiNardo: AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Agios: Honoraria, Research Funding. de Botton: Agios Pharmaceuticals, Inc.: Honoraria, Research Funding; Celgene Corporatation: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Servier: Honoraria. Tu: Celgene Corporation: Employment, Equity Ownership. Swern: Celgene Corporation: Employment, Equity Ownership. Tosolini: Celgene Corporation: Employment, Equity Ownership. Gupta: Celgene Corporation: Employment, Equity Ownership. Agresta: Agios Pharmaceuticals, Inc.: Employment, Equity Ownership. Fathi: Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.